Department Water Resources and Drinking Water
Dating with CFCs
CFCs are frquently used for dating young groundwater, see the project section for examples.
Text is taken from: Cook, P. G. and D. K. Solomon (1997) Recent advances in dating young groundwater: chlorofluorocarbons, 3H/3He and 85Kr. J. Hydrol., 191(1-4), 245-265.
Chlorofluorocarbons CFCs
Chlorofluorocarbons (CFCs) are man-made organic compounds which are produced for a range of industrial and domestic purposes (Rowland, 1990). Chlorofluorocarbons CFC-11 (CFCl3), CFC-12 (CF2Cl2) and CFC-113 (C2F3Cl3) have relatively long residence times in the atmosphere (44 years, 180 years and 85 years, respectively; Cunnold et al., 1994; Ko and Jackman, 1994), and so their atmospheric concentrations are uniform over large areas, and are steadily increasing. Concentrations of these CFCs in ocean basins have been used to study mixing processes, and the movement of deep ocean currents (Trumbore et al., 1991; Wallace et al., 1992). CFC concentrations in groundwater have been used to estimate groundwater age (Thompson and Hayes, 1979; Busenberg and Plummer, 1992; Dunkle et al., 1993).
Atmospheric concentrations
Concentrations of chlorofluorocarbons CFC-11, CFC-12 and CFC-113 in the atmosphere have been increasing steadily over the past 50 years (Figure 1).
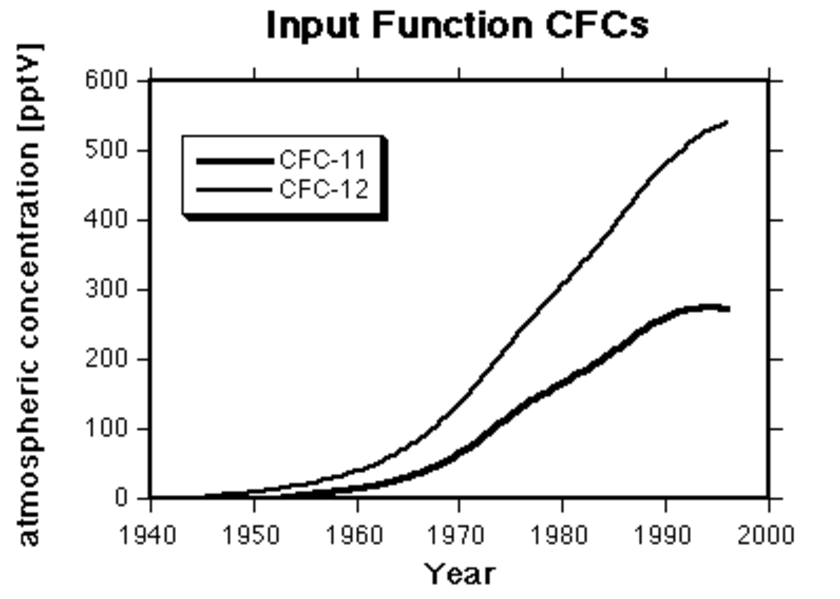
Measurements of atmospheric concentrations have been made since July 1978 at stations throughout the world as part of the Atmospheric Lifetime Experiment (Prinn et al., 1983; Cunnold et al., 1994; Fraser et al., 1996). (At the Pacific North West, USA, and South Pole stations, regular measurements of CFC-11 and CFC-12 have been made since 1975; Rasmussen and Khalil, 1986.) Atmospheric concentrations show little spatial variation, with only 10% variation observed between average concentrations in Ireland, Oregon, Barbados, Samoa and Tasmania (Cunnold et al., 1994). This is in strong contrast to the spatially variable nature of 3H concentrations in rainfall. For CFCs, the atmospheric input to the ground-water can be known with a high degree of precision, even at remote sites. Atmospheric concentrations for the period before their regular measurement have been reconstructed from estimates of world-wide production of chlorofluorocarbons and their rate of release to the atmosphere (McCarthy et al., 1977; AFEAS, 1993).
As a result of various regulations regarding the use of CFCs, production of CFC-11, CFC-12 and CFC-113 is declining. Production estimates for 1992 are approximately half of late 1980s peak values (AFEAS, 1993). Modelling suggests that atmospheric concentrations of CFC-11 and CFC-12 will reach a maximum before the turn of the century, after which they will slowly decline (Khalil and Rasmussen, 1993; Elkins et al., 1993). A similar scenario is likely for CFC-113. The sensitivity of the CFC dating method depends on the rate of change of the atmospheric CFC concentration with time, and thus the ability to date very young water will diminish with time. However, the ability to date groundwater that entered the saturated zone before the year 2000 will not change for several decades.
Method
Apparent CFC ages are obtained by converting measured CFC concentrations in groundwater to equivalent air concentrations using known solubility relationships (Warner and Weiss, 1985; Bu and Warner, 1995) and the recharge temperature. Corrections for excess air are made if appropriate (Busenberg and Plummer, 1992). These concentrations are compared with the atmospheric concentration curve (Figure 1) to obtain an apparent CFC age.
