Department Environmental Chemistry
Characterization of the Redox Properties of Iron Minerals by Combined Electrochemical and Spectroscopic Analyses
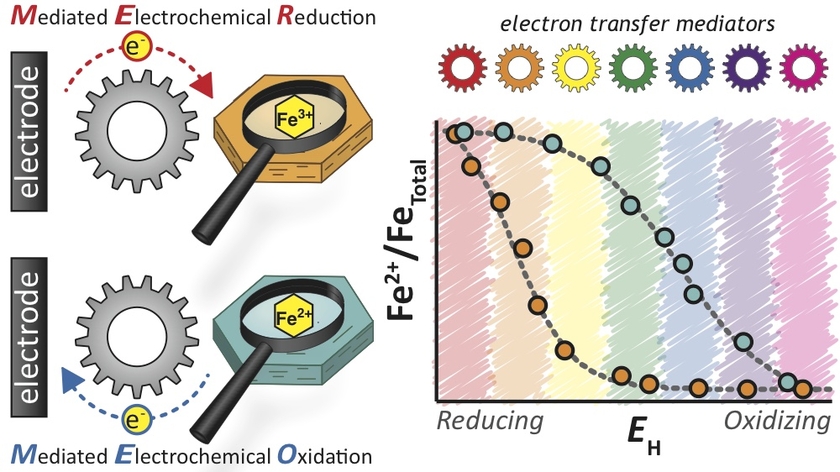
Electron transfer reactions involving Fe3+/Fe2+ couples associated with iron-bearing minerals play a key role in biogeochemical redox cycles and affect the availability, mobility, persistence, and toxicity of trace elements and many organic and inorganic contaminants. Despite their importance, the fundamental redox properties of these minerals are not well established.
The characterization of the redox properties of iron-bearing mineral is both conceptually and experimentally challenging due to the fact that electron transfer to/from Fe minerals is linked to other processes such as the adsorption and desorption of Fe2+, precipitation reactions, and mineral re-crystallization. In this project, we aim at overcoming these challenges by combining mediated electrochemical analysis with spectroscopic studies.
Latest publications
- Gorski, C. A.; et al. Redox Properties of Structural Fe in Clay Minerals: 3. Relationships between Smectite Redox and Structural Properties. Environ. Sci. Technol. 2013, 47, 13477–13485.
- Sander, M. et al. Electrochemical analyses of redox-active minerals: A review of non-mediated and mediated approaches, Environ. Sci. Technol. 2015, submitted
