Department Environmental Toxicology
Developing an in vitro fish cell-based model for neurotoxicity testing
The extensive use of chemicals and their release into water bodies pose a major environmental problem. Among other effects, these chemicals can affect the neurophysiology of aquatic organisms, thereby modifying their behavior and impairing their chances of survival. Environmental risk assessment of chemicals largely depends on expensive, time-consuming and ethically questionable animal experiments, often involving fish as test species. They moreover provide only limited understanding of the molecular mechanisms underlying the action of neurotoxicants and other chemicals. These factors point to an urgent need for better and faster alternatives for toxicity assessment. Here, fish cell-based in vitro approaches represent a promising technology for hazard identification of anthropogenic products and for the dissection of mechanisms leading to neuro- and other types of toxicity.
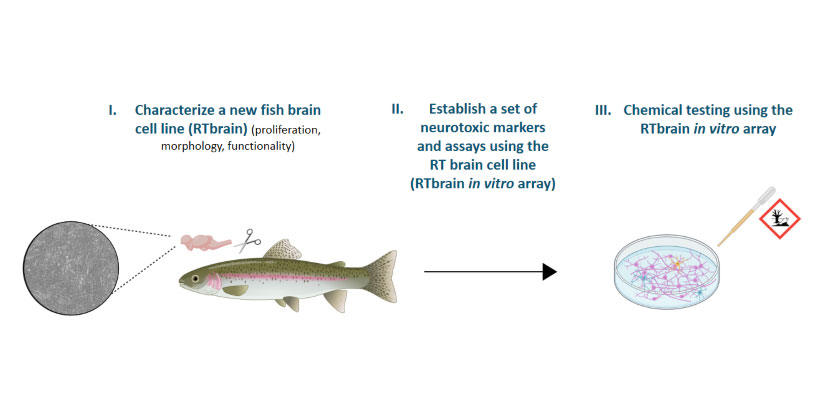
Part of the reason for the lack of acceptance of in vitro fish cell line assays for environmental risk assessment is the limited characterization of fish cell lines representative of toxicity-relevant tissues. With a focus on a cell line derived from the brain tissue of a rainbow trout (RTbrain), the aim of this project is therefore (i) the basic characterization of a range of potentially toxicologically relevant fish cell lines and (ii) the development of an assay to assess neurotoxicity based on RTbrain cells. This research is part of a larger project that aims to develop a modular, computationally-linked framework that integrates different fish cell lines and mechanistic endpoints to allow fit-for-purpose animal-free prediction of chemical toxicity to fish, which is funded by the Swiss National Research Program 79 “Advancing 3Rs – Research, Animals and Society”.[BJ2] Within this “Fish invitrome” framework, the RTbrain cell-based assay will represent the neurotoxicity module.
In the first part of the project, fish cell lines are studied with regard to their transcriptome and proteome, which will provide information on functional and molecular characteristics. RTbrain cells are more thoroughly investigated by immunostaining techniques and electrophysiological assessment to gain structural and functional information. The data generated with these studies will be used to assess candidate neurotoxicity targets based on which different neurotoxicity assays will be established. Eventually, a few assays will be selected and assembled into the RTbrain in vitro array, which is anticipated to measure neurotoxicity across a range of different neurologically relevant outcomes. Through chemical testing we will ultimately assess the robustness and predictivity of the RTbrain in vitro array.
With the expansion of the “Fish invitrome”, we expect to increase the value of new in vitro approaches in environmental risk assessment and facilitate adoption by regulators. The established fish brain module will ideally provide an animal-free method for high-throughput screening of data-poor compounds. In addition, knowledge will be gained of the basic molecular mechanisms of neurotoxicity.




